
The landscape of modern CNS drug development is marked by challenges including CNS complexity, BBB limitations, disease heterogeneity, biomarker scarcity, safety concerns, limited treatments, regulatory hurdles. Opportunities arise from neuroscience advancements, precision medicine, innovative drug delivery, biomarker development, neuroplasticity, digital health, collaborations, and patient-centric approaches, shaping the future of CNS therapeutics.
The field of Central Nervous System (CNS) drug development is both promising and complex, marked by a range of challenges and opportunities. Neurological disorders, with their intricate mechanisms and diverse manifestations, present formidable hurdles for researchers and pharmaceutical innovators. At the same time, advancements in neuroscience, technology, and personalized medicine offer unprecedented avenues for targeted therapies and improved patient outcomes. This exploration delves into the key challenges faced and the exciting opportunities unfolding in modern CNS drug development, shedding light on the dynamic landscape of neuropharmacology.
Modern Central Nervous System (CNS) drug development faces a myriad of challenges and opportunities that shape the landscape of neuropharmacology. Here's a detailed exploration of these aspects:
1. Complexity of the CNS:
The complexity of the Central Nervous System (CNS) presents a formidable challenge in understanding neurological disorders and crafting targeted therapies. At its core, this complexity stems from the intricate network of structures and functions within the brain and spinal cord. From governing sensory perception, motor control, and cognition to regulating emotions and memory, the CNS orchestrates a diverse array of functions. This diversity extends to the cellular level, where various types of neurons, glial cells, and specialized cells interact in complex ways, influencing neural communication and brain maintenance. Disruptions in neurotransmitter systems further complicate matters, as imbalances in key neurotransmitters like dopamine and serotonin are linked to disorders such as depression and schizophrenia. Adding to this complexity are genetic predispositions, environmental factors, and the brain's remarkable plasticity, which enables it to reorganize in response to stimuli or injuries. Protective barriers like the Blood-Brain Barrier (BBB) also play a role, safeguarding the CNS while posing challenges in drug delivery. Moreover, the clinical heterogeneity of neurological disorders, characterized by varying symptoms and treatment responses among patients, underscores the need for personalized approaches in diagnosis and therapy. In essence, navigating the complexities of the CNS requires a deep understanding of its multi-dimensional nature, from molecular mechanisms to clinical manifestations, to advance the development of effective CNS therapies.
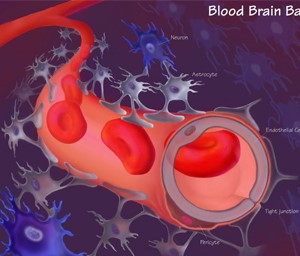
The Blood-Brain Barrier (BBB) serves as a critical defense mechanism for the Central Nervous System (CNS) by tightly regulating the passage of substances between the bloodstream and the brain. While this barrier protects the brain from harmful agents, it also presents a major hurdle in drug delivery for neurological disorders. The BBB's selective permeability limits the entry of many drugs, including potential therapeutics, into the brain parenchyma, where they can exert their intended effects. This poses a significant challenge for researchers and pharmaceutical developers, as it hampers the effectiveness of drugs targeting CNS disorders. Overcoming the BBB requires innovative strategies such as nanotechnology-based delivery systems, drug conjugates, or receptor-mediated transport mechanisms that can bypass or traverse this barrier effectively. Additionally, understanding the BBB's dynamic nature in health and disease is crucial for designing interventions that can enhance drug delivery while maintaining CNS homeostasis and minimizing off-target effects. Thus, the BBB stands as a key target for innovation in drug delivery technologies aimed at improving the efficacy of CNS therapeutics.

Neurological disorders exhibit a striking heterogeneity, spanning a spectrum of conditions with varying causes, manifestations, and clinical courses. This diversity poses a significant challenge in developing standardized treatments that effectively address the complexities of each disorder. Conditions such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, epilepsy, and neuropsychiatric disorders like schizophrenia and bipolar disorder illustrate the breadth of neurological diversity. Etiologies can range from genetic factors and neurodegenerative processes to autoimmune responses, infections, or traumatic injuries. Moreover, symptoms may manifest differently among individuals, leading to diagnostic complexities and personalized treatment requirements. This heterogeneity underscores the need for tailored, patient-centric approaches to neurological care. Precision medicine, leveraging genetic profiling, biomarkers, and advanced imaging techniques, offers promising avenues for stratifying patients based on their unique characteristics and optimizing treatment outcomes. By recognizing and addressing the diverse nature of neurological disorders, healthcare providers and researchers can strive towards more effective and personalized therapeutic strategies for patients across the neurological spectrum.

The absence of reliable biomarkers poses a significant challenge in the realm of Central Nervous System (CNS) disorders, impacting early diagnosis, treatment monitoring, and drug development efforts. Unlike some other medical conditions where biomarkers serve as clear indicators of disease presence or progression, many CNS disorders lack such definitive biological markers. This deficiency complicates the diagnostic process, often leading to delayed or missed diagnoses, which can significantly affect patient outcomes. Additionally, the absence of biomarkers hinders the ability to accurately monitor treatment responses and disease progression, making it challenging to assess the efficacy of therapeutic interventions in clinical trials. This lack of objective measures also contributes to the variability observed in patient responses to treatments, further complicating the development of standardized therapies. Addressing this challenge requires concerted research efforts to identify and validate biomarkers specific to various CNS disorders. Advances in neuroimaging techniques, molecular biology, and omics technologies offer promising avenues for discovering and utilizing biomarkers for early detection, prognostication, and treatment optimization in CNS disorders. By overcoming the hurdle of biomarker scarcity, researchers and clinicians can enhance diagnostic accuracy, tailor treatments more effectively, and accelerate the pace of drug development in the field of neurology.

The development of Central Nervous System (CNS) drugs is intricately tied to the delicate balance between efficacy and safety. While these drugs aim to alleviate symptoms and improve neurological conditions, they also carry inherent risks, particularly concerning their impact on cognition, mood, and behavior. Adverse effects in these domains can profoundly influence patient outcomes, affecting quality of life, treatment adherence, and overall well-being. The challenge lies in optimizing therapeutic benefits while minimizing potential risks and side effects associated with CNS medications.
For instance, drugs targeting psychiatric disorders must navigate the fine line between symptom relief and unwanted effects such as sedation, cognitive impairment, or mood destabilization. Similarly, medications for neurological conditions like epilepsy or Parkinson's disease need to strike a balance between controlling symptoms and avoiding adverse reactions that could impair daily functioning or exacerbate underlying conditions.
Addressing safety concerns in CNS drug development requires comprehensive preclinical and clinical assessments to evaluate potential risks and benefits. Robust pharmacovigilance strategies, post-marketing surveillance, and risk management plans are essential components of ensuring patient safety throughout the drug lifecycle. Additionally, advancements in pharmacogenomics, personalized medicine approaches, and novel drug delivery technologies offer opportunities to tailor treatments and mitigate safety risks based on individual patient profiles. By prioritizing safety alongside efficacy, CNS drug developers can enhance therapeutic outcomes while safeguarding patient well-being.
Certain neurological disorders, including specific types of dementia and Amyotrophic Lateral Sclerosis (ALS), continue to present a significant challenge in terms of limited treatment options and a lack of effective disease-modifying therapies. These conditions underscore the pressing unmet medical needs within the realm of neurology. Despite advancements in medical science and therapeutic interventions, disorders like Alzheimer's disease, vascular dementia, frontotemporal dementia, and ALS remain elusive in terms of treatments that can alter disease progression or provide substantial long-term benefits.
The complexity of these neurological conditions, coupled with the intricate mechanisms underlying their pathogenesis, contributes to the difficulty in developing targeted and efficacious therapies. Alzheimer's disease, for example, involves multifactorial processes such as beta-amyloid accumulation, tau protein pathology, neuroinflammation, and synaptic dysfunction, making it challenging to identify single-target interventions that can halt or reverse the disease course. Similarly, ALS is characterized by progressive motor neuron degeneration, yet effective strategies to slow or stop this degeneration are limited.
The lack of disease-modifying treatments not only impacts patient outcomes but also emphasizes the critical need for ongoing research, clinical trials, and collaborative efforts in neurology. Innovative approaches, including gene therapies, stem cell therapies, neuroprotective agents, and precision medicine strategies tailored to individual disease subtypes, hold promise for addressing these unmet medical needs. Furthermore, initiatives that promote early diagnosis, multidisciplinary care, and patient advocacy play crucial roles in improving quality of life and outcomes for individuals living with these challenging neurological conditions.

Stringent regulatory requirements and lengthy approval processes pose significant hurdles in the development of novel Central Nervous System (CNS) therapies. These regulatory challenges contribute to increased timeframes and costs associated with bringing new treatments to market, especially in the field of neuropharmacology.
The regulatory landscape for CNS drugs is characterized by rigorous standards aimed at ensuring safety, efficacy, and quality. However, these standards often necessitate extensive preclinical studies, robust clinical trials, and comprehensive data submissions, leading to prolonged development timelines and substantial financial investments. Furthermore, the complexity of CNS disorders, with their diverse symptoms, variable disease progression, and heterogeneous patient populations, adds layers of complexity to regulatory evaluations.
For drug developers, navigating these regulatory hurdles involves meticulous planning, adherence to regulatory guidelines, and collaboration with regulatory agencies to streamline processes where possible. Innovative trial designs, such as adaptive trials and real-world evidence studies, are increasingly being utilized to expedite data collection and regulatory reviews. Additionally, engaging in early discussions with regulatory authorities, incorporating patient perspectives, and leveraging technological advancements in data analysis and pharmacovigilance can help address regulatory challenges more effectively.
While regulatory requirements play a crucial role in ensuring patient safety and product efficacy, finding a balance between regulatory compliance and expeditious drug development remains a key priority in advancing novel CNS therapies to address unmet medical needs and improve patient outcomes.
1. Advancements in Neuroscience:
Advancements in neuroscience are revolutionizing our understanding of Central Nervous System (CNS) disorders and driving innovation in target identification and drug discovery. Ongoing research efforts encompass diverse areas such as neuroimaging, molecular biology, and genetics, collectively contributing to deeper insights into the intricate mechanisms underlying neurological conditions.
Neuroimaging techniques, such as functional MRI (fMRI), positron emission tomography (PET), and diffusion tensor imaging (DTI), offer unprecedented visualization of brain structure, function, and connectivity. These imaging modalities enable researchers to map neural networks, identify biomarkers of disease progression, and assess treatment effects in real-time, providing invaluable data for refining therapeutic strategies.
At the molecular level, advancements in molecular biology techniques, including genomics, proteomics, and transcriptomics, have unraveled key molecular pathways implicated in CNS disorders. Genetic studies have identified susceptibility genes, rare variants, and epigenetic modifications associated with conditions like Alzheimer's disease, Parkinson's disease, and autism spectrum disorders, offering new avenues for targeted therapies and personalized medicine approaches.
Furthermore, insights from genetics and molecular biology have paved the way for innovative drug discovery strategies, such as precision medicine and gene therapies. Target identification, validation, and drug screening processes benefit from a deeper understanding of disease mechanisms at the molecular and cellular levels, accelerating the development of novel therapeutics with enhanced efficacy and specificity.
In summary, the continuous progress in neuroscience, driven by advancements in neuroimaging, molecular biology, and genetics, is transforming the landscape of CNS research and drug development. These interdisciplinary efforts not only expand our knowledge of CNS disorders but also hold promise for translating scientific discoveries into tangible clinical interventions that improve patient outcomes and quality of life.
2. Precision Medicine:

The emergence of precision medicine represents a paradigm shift in healthcare, particularly in the realm of Central Nervous System (CNS) disorders. Precision medicine leverages advancements in genetics, molecular biology, and data analytics to customize treatments according to individual genetic, molecular, and clinical profiles. This personalized approach holds immense promise for improving therapeutic outcomes while mitigating side effects in CNS disorders.
By analyzing a patient's genetic makeup, including variations in genes associated with CNS conditions, precision medicine enables clinicians to identify genetic factors contributing to disease susceptibility, progression, and treatment response. This genetic information, combined with molecular profiling techniques such as biomarker analysis and omics technologies, allows for precise stratification of patients into subgroups with distinct molecular signatures. These subgroups may respond differently to therapies, necessitating tailored treatment approaches for optimal outcomes.
Moreover, precision medicine extends beyond genetics to encompass clinical characteristics such as disease stage, comorbidities, biomarker levels, and response to previous treatments. Integrating these multidimensional data points through advanced computational algorithms and machine learning algorithms enables clinicians to develop personalized treatment regimens tailored to each patient's unique needs and characteristics.
The benefits of precision medicine in CNS disorders are manifold. It facilitates the selection of targeted therapies that address specific molecular pathways implicated in disease pathogenesis, enhancing treatment efficacy and reducing the likelihood of adverse reactions. Additionally, by optimizing treatment strategies based on individual profiles, precision medicine contributes to more efficient healthcare resource utilization and improved patient satisfaction.
Overall, the rise of precision medicine heralds a new era of personalized care in CNS disorders, offering hope for optimized treatments, better therapeutic outcomes, and ultimately, improved quality of life for patients.
3. Drug Delivery Innovations:

Drug delivery innovations are transforming the landscape of Central Nervous System (CNS) therapies by overcoming the challenges posed by the Blood-Brain Barrier (BBB). Nanotechnology, drug conjugates, and targeted delivery systems are at the forefront of these advancements, revolutionizing drug delivery across the BBB, enhancing efficacy, and reducing systemic toxicity.
Nanotechnology involves the design and fabrication of nanoscale drug carriers, such as nanoparticles and liposomes, capable of encapsulating therapeutic agents. These nano-sized carriers offer several advantages, including increased drug stability, prolonged circulation time, and enhanced BBB penetration due to their small size and surface modifications. By encapsulating CNS drugs within nanocarriers, researchers can improve drug solubility, bioavailability, and brain tissue distribution, leading to enhanced therapeutic effects.
Drug conjugates represent another innovative approach wherein drugs are chemically linked to carrier molecules that facilitate BBB transport. These conjugates can exploit endogenous transport mechanisms or receptor-mediated pathways to bypass the BBB's restrictive barrier and deliver drugs directly to their target sites within the CNS. This targeted delivery minimizes off-target effects and systemic toxicity, improving the overall safety profile of CNS therapies.
Targeted delivery systems utilize ligands or antibodies that specifically bind to receptors or transporters expressed on BBB endothelial cells. By leveraging these targeted interactions, drug-loaded carriers can selectively cross the BBB and accumulate in diseased brain regions, maximizing therapeutic efficacy while minimizing exposure to healthy tissues. Additionally, advancements in nanotechnology allow for controlled release mechanisms, enabling sustained drug release and prolonged therapeutic effects.
These drug delivery innovations hold immense potential for addressing unmet medical needs in CNS disorders, including neurodegenerative diseases, brain tumors, and neuroinflammatory conditions. By enhancing drug efficacy, reducing systemic side effects, and enabling precise targeting of diseased brain regions, these technologies contribute to the development of safer, more effective CNS therapies that improve patient outcomes and quality of life.
4. Biomarker Development:

Advancements in biomarker research are revolutionizing the field of Central Nervous System (CNS) disorders by enabling early diagnosis, patient stratification, and monitoring of treatment response. Biomarkers, which encompass a wide range of molecular, genetic, imaging, and biochemical indicators, play a crucial role in accelerating clinical development and fostering personalized medicine approaches.
One of the primary benefits of biomarker development is early disease detection. Biomarkers can detect subtle changes in biological processes associated with CNS disorders even before clinical symptoms manifest, enabling proactive interventions and disease management strategies. Early diagnosis not only improves patient outcomes but also provides a window of opportunity for implementing targeted therapies at a stage when they are most effective.
Biomarkers also facilitate patient stratification based on disease subtypes, molecular profiles, and treatment responsiveness. This stratification allows clinicians to tailor treatment regimens to individual patient characteristics, maximizing therapeutic benefits while minimizing adverse effects. Moreover, biomarker-guided patient selection in clinical trials enhances trial efficiency, increases the likelihood of demonstrating treatment efficacy, and accelerates the development of novel therapies.
In addition to aiding diagnosis and patient stratification, biomarkers play a vital role in monitoring treatment response and disease progression. They provide objective measures of therapeutic efficacy, allowing clinicians to adjust treatment plans in real time and optimize patient outcomes. Biomarker-based monitoring also supports the development of predictive models and algorithms for forecasting disease trajectories, guiding long-term management strategies.
Overall, advancements in biomarker research are transforming CNS healthcare by facilitating early detection, personalized treatment approaches, and precision medicine interventions. By leveraging biomarkers for early diagnosis, patient stratification, and treatment monitoring, clinicians and researchers can accelerate clinical development, improve therapeutic outcomes, and ultimately enhance the quality of life for individuals affected by CNS disorders.
5. Neuroplasticity and Neuroregeneration:
The concepts of neuroplasticity and neuroregeneration represent promising avenues in the quest for disease-modifying therapies, especially in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Neuroplasticity refers to the brain's ability to reorganize its structure and function in response to experiences, learning, and environmental stimuli. Neuroregeneration, on the other hand, involves the growth and restoration of neural connections, neurons, and brain tissue.
Understanding neuroplasticity and neuroregeneration offers insights into the mechanisms underlying CNS disorders and provides opportunities to develop interventions that can modify disease progression. In neurodegenerative conditions where neuronal loss and synaptic dysfunction are hallmark features, harnessing neuroplasticity and promoting neuroregeneration are key strategies to counteract neuronal damage and functional decline.
For instance, research in Alzheimer's disease focuses on stimulating neuroplasticity mechanisms to enhance cognitive reserve, promote synaptic plasticity, and potentially slow cognitive decline. Techniques such as cognitive training, environmental enrichment, and brain stimulation therapies aim to capitalize on the brain's inherent plasticity to preserve cognitive function and delay disease progression.
In Parkinson's disease, efforts are directed towards promoting neuroregeneration by targeting dopaminergic neurons and restoring dopamine levels in the brain. Stem cell therapies, growth factors, and gene therapies are being explored to regenerate damaged neurons, improve motor symptoms, and potentially modify the course of the disease.
Moreover, advancements in neuroimaging technologies allow researchers to visualize neuroplasticity and neuroregeneration in vivo, enabling monitoring of treatment effects and disease progression. Biomarkers associated with synaptic integrity, neurogenesis, and neuronal connectivity serve as indicators of neuroplasticity and regeneration, guiding the development and assessment of novel therapies.
Overall, leveraging neuroplasticity and neuroregeneration holds immense promise for developing disease-modifying treatments in neurodegenerative disorders. By harnessing the brain's capacity for adaptive change and regeneration, researchers aim to not only alleviate symptoms but also address the underlying pathological processes, paving the way for more effective and transformative therapies in CNS healthcare.
6. Digital Health Technologies:

The integration of digital health technologies represents a significant advancement in healthcare, particularly in the management of Central Nervous System (CNS) disorders. Digital health tools, including wearables, mobile applications, and AI-driven diagnostics, play a crucial role in enabling remote patient monitoring, real-time data collection, and personalized treatment optimization.
Wearables, such as smartwatches and fitness trackers, equipped with sensors for tracking vital signs, activity levels, and sleep patterns, provide continuous monitoring of patients' health status. In CNS disorders like epilepsy, wearables can detect seizure activity, alert caregivers or healthcare providers, and facilitate timely interventions. Similarly, in neurodegenerative diseases like Parkinson's, wearables can monitor movement disorders, medication adherence, and disease progression, enabling personalized adjustments to treatment regimens.
Mobile applications designed for CNS disorders offer various functionalities, including medication reminders, symptom tracking, mood monitoring, and cognitive exercises. These apps empower patients to actively participate in their healthcare management, enhance treatment adherence, and provide valuable data for healthcare providers to make informed decisions. For instance, apps that use cognitive behavioral therapy techniques can support individuals with anxiety disorders or depression, offering personalized interventions and real-time feedback.
AI-driven diagnostics leverage machine learning algorithms to analyze large datasets of clinical, imaging, and genetic information, aiding in disease diagnosis, prognosis, and treatment planning. In CNS disorders, AI algorithms can assist in interpreting neuroimaging scans, identifying biomarkers of disease progression, and predicting treatment responses. This data-driven approach enhances clinical decision-making, reduces diagnostic errors, and promotes personalized treatment strategies tailored to each patient's unique profile.
Overall, digital health technologies revolutionize CNS healthcare by facilitating continuous monitoring, data-driven insights, and personalized interventions. By harnessing the power of wearables, mobile apps, and AI-driven diagnostics, healthcare providers can optimize patient outcomes, improve quality of life, and advance precision medicine approaches in the management of CNS disorders.
7. Collaborative Research Initiatives:

Collaborative research initiatives are pivotal in driving advancements in Central Nervous System (CNS) therapeutics by fostering synergistic partnerships between academia, industry, and regulatory agencies. These collaborative efforts facilitate innovation, promote data sharing, and streamline drug development processes, ultimately accelerating progress in the field of CNS healthcare.
Academic institutions contribute valuable scientific expertise, research infrastructure, and clinical insights to collaborative initiatives. Researchers in academia conduct fundamental studies, elucidate disease mechanisms, and explore novel therapeutic targets in CNS disorders. Their contributions lay the groundwork for translational research and therapeutic innovation, providing a strong scientific foundation for drug development efforts.
Industry partners, including pharmaceutical companies, biotechnology firms, and medical device manufacturers, bring essential resources, technological capabilities, and investment into collaborative research initiatives. Industry-academic collaborations enable the translation of scientific discoveries into clinical applications, facilitate preclinical and clinical trials, and expedite the commercialization of promising therapies. Industry expertise in drug development, regulatory compliance, and market access complements academic research efforts, fostering a seamless transition from bench to bedside.
Regulatory agencies play a critical role in collaborative research initiatives by providing guidance, oversight, and regulatory pathways for new CNS therapies. Collaborative interactions between researchers, industry stakeholders, and regulatory authorities promote transparency, facilitate data exchange, and ensure compliance with regulatory standards. This collaborative approach streamlines the drug approval process, accelerates timelines for clinical trials, and enhances patient access to innovative CNS treatments.
Moreover, collaborative research initiatives promote knowledge sharing, data harmonization, and best practices across diverse stakeholders, fostering a culture of innovation and continuous improvement in CNS therapeutics. By leveraging collective expertise, resources, and networks, collaborative efforts drive meaningful advancements in disease understanding, treatment efficacy, and patient outcomes in the field of CNS healthcare.
8. Patient-Centric Approaches:
Patient-centric approaches are fundamental to advancing Central Nervous System (CNS) therapeutics by prioritizing patient advocacy, engagement, and input throughout the drug development process. These approaches ensure that treatments align with patient needs, preferences, and quality of life considerations, ultimately leading to improved therapeutic outcomes and enhanced patient satisfaction.
Patient advocacy organizations play a crucial role in representing the interests and perspectives of individuals affected by CNS disorders. They advocate for increased awareness, research funding, access to treatments, and supportive services for patients and their families. By amplifying patient voices, advocacy groups influence policy decisions, research priorities, and healthcare initiatives, driving positive changes in CNS healthcare delivery.
Patient engagement is another cornerstone of patient-centric approaches, involving patients as active participants in decision-making, research design, and treatment planning. Collaborative partnerships between patients, healthcare providers, researchers, and industry stakeholders ensure that patient perspectives are integrated into every stage of drug development, from study design to regulatory approval and post-market surveillance.
Patient input is invaluable in shaping clinical trial protocols, outcome measures, and treatment endpoints that are meaningful and relevant to patients' lived experiences. Patient-reported outcomes (PROs), patient preference studies, and qualitative research methodologies capture patient feedback, preferences, and treatment goals, informing the development of patient-centered therapies that address real-world needs and priorities.
Furthermore, patient-centric approaches promote transparency, communication, and shared decision-making between patients and healthcare providers. Empowering patients with information, education, and resources fosters a collaborative care model where patients are active partners in managing their health, making informed treatment choices, and advocating for their well-being.
In summary, patient-centric approaches in CNS therapeutics prioritize patient advocacy, engagement, and input, ensuring that treatments are tailored to meet patient needs, preferences, and quality of life considerations. By placing patients at the center of drug development efforts, these approaches drive meaningful improvements in patient outcomes, treatment satisfaction, and overall healthcare delivery in the CNS field.
Addressing these challenges and leveraging opportunities in modern CNS drug development requires interdisciplinary collaboration, innovative technologies, regulatory flexibility, and a patient-centered approach to ultimately improve outcomes for individuals with neurological disorders.