
Every living cell, including cancer, depends on metabolism. The metabolic dysfunction associated with cancer was recognized nearly 100 years ago by Otto Warburg and is now recognized as an important hallmark of the disease. In addition, metabolic alterations have been reported as an important event during early stages of cancer progression. In precision medicine, drugs designed to block altered metabolic functions are integrated, but their clinical effectiveness remains uncertain. Metabolic targeted therapeutic paradigms are still evolving, and this opinion discusses the challenges of developing and implementing these drugs.

Although genes encode cellular functions and fates, with DNA acting as an inert cookbook, metabolism is the lifeblood of every living cell. Every living cell is governed by metabolic processes that consist of thousands of biochemical reactions that serve as architects, supply chains, energy sources, chefs, signaling and timekeepers, etc. Consequently, healthy living is characterized by a physiologically well-regulated metabolic state, and pathological diseases are characterized by metabolic imbalances. Disease symptoms are primarily manifestations of metabolic disturbances occurring within sick or affected cells. As a result, clinical biochemistry is an essential part of modern medicine for diagnosing and treating most diseases, such as diabetes, metabolic disorders, oncology, cardiology, neurology, organ failures, allergies, infectious diseases, and various genetic conditions. The fact that metabolic changes are closely related to diseased states makes it vital that drugs used for disease treatment do not disrupt normal metabolism. It is therefore essential that metabolic investigations focused on mechanism of action, safety assessments, and pharmacokinetics and pharmacodynamic properties are conducted in pharmacological drug discovery and development so that FDA approval can be gained for every new drug to be introduced to the clinical setting.
Metabolic dysregulation is a hallmark of cancer. It is important to recognize that cancer is a disease characterized by uncontrolled proliferation. It is impossible for any living cell, including malignant, to survive or proliferate without metabolic support. When it comes to pharmacological interventions that target cancer metabolism, a better understanding of the differences between physiological metabolisms and (cancer) pathological metabolic imbalances is necessary, as well as the interplay between them. The development of metabolic targeted therapy for cancer depends on establishing these fundamental concepts, and this perspective discusses some of these fundamental concepts as well as progress and challenges facing the clinical development of cancer therapies that disrupt dysregulated metabolism.
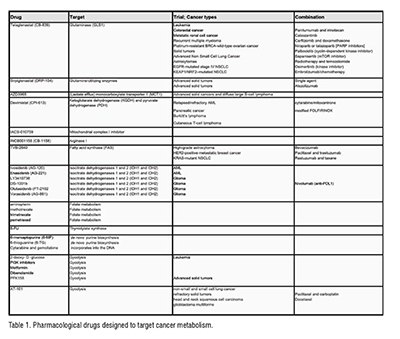
All living creatures of this nature rely on metabolism for survival. From our understanding about the biological relationships between plant and animal cells, it becomes clear that carbon dioxide, water, oxygen, and glucose are essential for respiration, energy transduction, as well as are the fundamental metabolites necessary for cell division and growth. Pentose sugars, derived from glucose, serve as the backbone upon which genetic codes are imprinted, preserved, and propagated. Consequently, glucose metabolism is the most vulnerable function that can be altered to initiate and spread cancerous growth. Accordingly, Otto Warburg's first and foremost fascinating discovery, which laid the foundation for cancer biology in 1923, revealed that cancer cells require glucose and oxygen (aerobic glycolysis), but he also observed strange lactate production, which is only expected to occur when glucose is metabolized without oxygen. Despite this long-standing recognition, therapeutics targeting metabolic functions in cancer only began to emerge in the past few decades. A major reason for the slow progress in discovery and development of cancer metabolism-targeting drugs is that the biochemical principles behind the "Warburg effect" remain obscure. In addition, enzymes that participate in metabolic pathways use complex biochemical principles, so changing the metabolic activity by mutation or overexpression alone is not sufficient. As metabolic activity is influenced by feedforward activation, feedback inhibition, post-translational modifications, concentrations of substrates, products, and cofactors, it is hard to understand these dynamics. Although metabolic imbalances are directly known to contribute to cancerous growth, recent research has shown that these imbalances also become important barriers through which cancer cells escape natural antitumor immune activity and enable developing resistance to standard treatments and immunotherapy. When it became apparent that altered metabolism interfered with the effectiveness of standard treatments (chemo/radiotherapy), targeted therapies (small molecules or immunotherapy), drug development strategies changed. As a result, several novel agents targeting glucose and metabolic functions were developed and tested either as single agent or in combination. A review of clinical evidence on such drugs aimed at targeting metabolism from the past few decades indicates that drugs that directly inhibit nucleic acid synthesis (methotrexate, 5-FU, pemetrexed, cytarabine, gemcitabine, etc.,) showed greater efficacy than drugs that targeted glucose/glutamine metabolism enzymes (see Table 1). So far, direct targeting of glycolytic, citric acid cycle and glutamine metabolic enzymes has yielded mixed results, but some promising results are being seen as drug combinations are being investigated in clinics (see Table 1). On the biological level, cancerous growth is indeed influenced by metabolic modifications, but so far, therapeutic interventions targeting these mechanisms have not yielded dramatic results. Although disappointing, it is crucial to understand that our incomplete biological knowledge is the greatest challenge, rather than pointing fingers at rationale for targeting metabolic functions in cancer treatment.
For a better understanding of why targeting metabolic pathways in the clinic is not yielding the therapeutic benefits expected and what needs to be done to improve on these, one must be able to take a holistic view of metabolic networks as they relate to signaling and functional pathways. It would be unfair to compare metabolic inhibitors that induce DNA damage to inhibitors designed to interrupt metabolic pathways. As unrepaired DNA damage is lethal for cell survival and interrupted metabolic pathways and/or energy deprivation would simply stop cells from dividing, these differences are explained partly by their mechanisms of action. Additionally, metabolic inhibitions could be overcome through anaplerosis (backfilling metabolic reactions), exchange of metabolites between nearby or distant cells, metabolic compensation, or pathway rewiring, which are all dynamically adaptable processes. Therefore, in drug discovery, it is crucial to identify the Achilles' heels of metabolism and develop appropriate targeting and or co-targeting strategies to ensure that compensatory metabolic and signaling responses are mitigated. Therefore, prior to even planning the path for target discovery, it is necessary to have comprehensive knowledge or conduct appropriate research to bridge gaps with existing knowledge. Once the drug has been developed, in addition to assessments of its targeted mechanism and cancer, further rigorous investigations must be conducted to identify the effects on global metabolic and signaling pathways, and this knowledge must be gathered before clinical trials can begin.
Although our knowledge of cancer biology is incomplete, a compendium of molecular and metabolic basis of disease progression in cancer does not exist either. In spite of rocket science being very complex, its success is largely due to the establishment and availability of blueprints from design to launch, collective responsibility shouldered by exhaustive collaborative efforts from diverse disciplines. It is unfortunate that disorganized research and the loss of unrecognized meaningful discoveries prevent millions of lives each year from being saved from cancer. While life-saving cancer research is progressing and better treatments such as precision medicine are becoming available, death rates may seem to be declining, but the sad truth is that the total number of deaths continues to rise. Precision medicine is therefore crucially dependent on improving our understanding of cancer progression at both molecular and biological levels. Cancer is generally considered to be a heterogeneous disease, but recent studies have shown that many different cancer types have linear biological patterns throughout their progression. Among these discoveries, genes associated with metabolism (Citric acid cycle, oxidative phosphorylation and electron transport chain) are found to increase at very early stages of cancer progression. Further genes associated with glucose, nucleotide and lipid metabolism were also found to be highly elevated in progressed cancers. As metabolism is deeply interconnected with virtually every cell function, simply accelerating that function has a profound impact on the ability of cancer cells to proliferate, resist treatment and immune responses, and spread rapidly. This makes metabolism a powerful force to be reckoned with when it comes to drug discovery. An overview of the impact of metabolism on major cellular functions is provided in Figure 1.
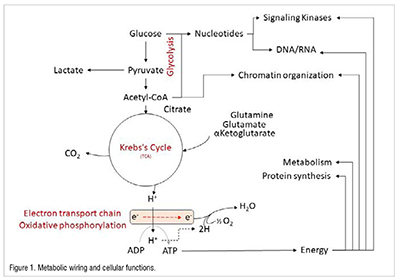
The increased glucose metabolism associated with cancer is a fundamental biological property, which led to the establishment of fluorodeoxyglucose (FDG) positron emission tomography (PET) as the most reliable tool in cancer diagnostics. While oxidation of glucose is the primary source of energy, cancer cells use amino acids such as glutamine/glutamic acid as alternate energy fuels, reducing the burning of glucose, which causes lactate production as observed by Otto Warburg. According to recent studies, these metabolic changes are merely meant to facilitate the redirection of glucose's carbon backbone into nucleotide synthesis. In malignancy, the synthesis of nucleotides may appear intuitively as a catalyst for facilitating DNA and RNA synthesis and promoting cell proliferation. However, nucleotides are more versatile molecules, carrying high energy transfers and facilitating thousands of reactions within the cells. Nucleotides exert enormous biological influence through their participation in 3494 metabolic reactions, their roles as cofactors in proteins (996) and protein complexes (1007), and their roles as reactants/messengers in signaling pathways that activate or inhibit 668 pathways (Figure 2). In addition, breakup analyses of major nucleotides show that ATP, a component of 636 metabolic reactions and 300 protein complexes, is essential to a variety of cellular functions, including DNA/RNA synthesis, repair, organ function, cell death, metabolic function, signaling function, and transport function (Figure 2). In addition, GTP, UTP, and CTP play an important role in macromolecular synthesis, transport, signaling, as well as metabolism (Figure 2).

In conclusion, the magnitude of biological influences impacted by nucleotides, which themselves come from a very modest carbon source, namely glucose, indicates why cancer cells attach such importance to the efficient handling of glucose. Blocking glucose (or dependent) metabolism, which is necessary both for cell survival and death, puts cancer cells in double jeopardy and therefore spares them the killing effects induced by metabolism-targeted therapies. As a result, most metabolic blockades simply prevent cells from dividing, causing them to remain suspended for prolonged periods of time, until conditions are reversed, when the cells can begin dividing again. There has been evidence that such effects can be observed in animal tumor models treated with glibenclamide (antidiabetic drug) (unpublished data), and as reported in several studies involving metformin, etc., In designing rationale drug combinations, such important lessons can help us apply metabolic principles to create effective therapies. As an example, we showed that depleting nucleotides through inhibiting a vital lipid metabolism improved 5-fluorouracil's (chemotherapeutic) cell killing activity in lymphoma, which otherwise would not be active. To summarize the current status of metabolic targeted cancer treatments, the development and design of drugs has been phenomenally successful, but the clinical outcomes of implementing these therapeutics as cancer treatments are surprisingly less impressive. Several roadblocks exist, including gaps in knowledge accumulated so far on understanding the wholistic nature of metabolism, its ramification with cellular pathways, how these are dynamically rewired, etc., as cancer progresses, has not been clearly established. As metabolic or signaling systems use complex networks of pathways, if blocked, they can take alternative routes. That makes it extremely challenging to develop appropriate therapeutic strategies. The challenges associated with understanding and navigating biological dynamics can be overcome by possibly integrating artificial intelligence in a way that enhances accuracy and provides benefits in clinical care in the future.
References:
1. Ravi D et.al, Front. Oncol., 26 Oct 2021, https://doi.org/10.3389/fonc.2021.725137
2. Ravi D. et.al, Biomedicines 24 Oct 2022, https://doi.org/10.3390/biomedicines10112720
3. Lemberg KM et.al, J Clin Invest, 4 Jan 2022, doi: 10.1172/JCI148550
4. Zaal EA et.al, Front. Oncol., 02 Nov 2018 https://doi.org/10.3389/fonc.2018.00500