
Pharmaceutical 3D printing is a rapidly growing field, with more clinical studies starting every year. Able to manufacture novel dosage forms with advanced release profiles personalised to the patient, this novel manufacturing technique is set to massively disrupt the pharmaceutical industry.

3D printing is a well-known additive manufacturing technique of the modern age. Since the first documentation of the process in 1980s Japan, 3D printing has entered many different fields, such as building construction, the arts and sports equipment. In healthcare, 3D printing has been used since the 1990s to manufacture dental implants and prosthetics, allowing for custom shape and size for each individual patient. More recently, 3D printing has entered the field of tissue engineering to build complex geometries out of novel biomaterials to mimic tissues. The use of 3D printing to manufacture medication, or pharmaceutical 3D printing, started growing in the early 2010s, pushed by leading academics attempting to make novel dosage forms fit complex and personalised patient needs. This new, disruptive approach to manufacture medication brings many advantages to healthcare and is set to revolutionise the pharmaceutical industry.
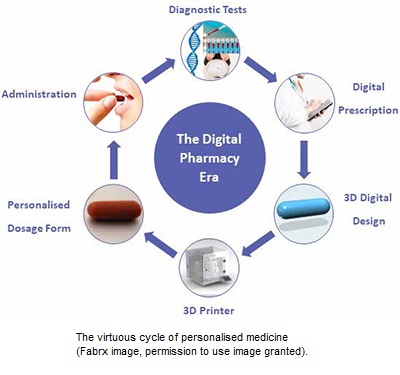
Pharmaceutical 3D printing has many different benefits over standard manufacturing techniques for medicines. Pharmaceutical 3D printing allows for the easy creation of hard-to-reach or complex release profiles. For example, fast oral disintegrating printlets (3D printed tablets) or multi-release profile polypills. Pharmaceutical 3D printing can be used to personalise medication, printing small batches of patient-specific prescriptions at a time with personalised dosages, colours, flavours, release profiles, shape and drug combinations as potential options. The small batch manufacture can also benefit clinical trials, reducing costs and waste associated with clinical trial batch manufacture and allowing for the rapid alteration of dosage and formulation when required.
There are many different 3D printing technologies being investigated and utilised to manufacture medication, each with different benefits and target uses. Below we take a closer look at small batch and mass manufacturing 3D printing as well as the techniques currently being exploited for them.
The World Health Organisation, European Medical Agency, and US National Institute of Health have all recognised the need for personalised dosage forms, tailored using patient data (age, weight, co-morbidities, gender, ethnicity, etc). $528 billion could be saved annually in the US through the increased treatment efficacy and reduced side effects that would be obtained with personalised medicine. The current method to personalise medicine, compounding, is slow, costly and prone to errors. An average of 41 patients are affected negatively for every compounding error, with the most infamous being the Framingham contamination error medication in 2012, effecting 13,534 patients with 753 documented instances of patient harm and 64 deaths, leading to regulatory reform in the US. However, personalised medicine preparation practices themselves are still outdated and often based on inaccurate dosing and carried out by hand. Pharmaceutical 3D printing could bring the compounding of oral medication into the modern, automated age. Money and time could also be saved if pharmaceutical 3D printing was implemented in clinical trial batch manufacture, allowing for the rapid production of novel dosage forms in smaller more manageable batches than currently feasible without preparation by hand.
Currently, pharmaceutical 3D printing can be used as a compounding manufacturing method, in line with compounding regulation. Leading regulatory agencies, including the FDA and MHRA, are now setting up legislation to allow for pharmaceutical 3D printing to be used for personalised medicine and small batch manufacture on a wider scale. Often termed point-of-care-manufacturing, once finalised this legislation will regulate hub and spot style systems with multiple printers in multiple hospitals and pharmacies being regulated by single hub organisations.
Extrusion based 3D printing technologies are best suited for personalised medicine and small batch manufacture, due to the versatility these techniques offer in terms of personalisation options. You can easily change the formulation colour, flavour and printed shape to fit the patients’ needs, or combine multiple formulations into a multi-layered, multi-drug polypill. For this reason, these technologies are the ones entering clinical studies for personalised medicine, but other techniques are also being pushed by eager academics looking to expand the field further.
Fused deposition modelling is one of the most well-known extrusion-based 3D printing technologies. A thermoresponsive filament made up of excipients and drugs, manufactured using hot melt extrusion, is fed through the printhead, melted and deposited on the build plate. As the layers are set on cooling, the 3D model can be built up. Fused deposition modelling allows for increased personalisation options compared to vat or bed techniques described above. The popularity of fused deposition modelling also adds to its attractiveness, having a wealth of literature behind the technique. The downside of fused deposition modelling is that drugs need to be thermally stable at temperatures used, which tend to be over 80 °C. Also, sometimes filament creation via hot melt extrusion can be tricky, with mechanical property issues preventing pharmaceutical filaments from entering the market. To tackle this, M3DISEEN.com was launched in 2021 by FABRX as a research-led, freely available, AI-driven, 3D printability prediction tool to aid filament creation and formulation development for fused deposition modelling.
Direct powder extrusion is another extrusion-based 3D printing technology and is one of the more novel approaches for 3D printing. Based on hot melt extrusion technology, a powder mix (drug and excipients) is fed directly into a hopper on the printhead. The powder mix is melted and pulled down via a rotating screw system to be deposited on the build plate, skipping the filament manufacturing step of fused deposition modelling. Because of this, direct powder extrusion is often seen as superior compared to fused deposition modelling as it shortens and simplifies the manufacture of printlets. Thermally stable drugs and excipients are required as melting is involved, but the simplicity of this system is proving attractive to pharmacists and formulation scientists entering the field. Direct powder extrusion can be used for personalised medicine on smaller scales or converted into a mass manufacturing method. FABRX is pushing ahead with the former with their M3DIMAKER pharmaceutical 3D printer series with some exciting hospital collaborations across Europe.
Semi-solid extrusion is another popular extrusion-based 3D printing technology. As the name suggests, semisolid extrusion uses gels or pastes containing the drug to build a 3D model. The semisolid mixture is pushed through the nozzle and deposited layer by layer on the build plate. Different methods of pushing the semi-solid through the printhead can be used, such as a stepper motor or compressed air. The semi-solid then sets via cooling or drying depending on the developed formulation. Published in 2019, the first and so far, only published clinical study for 3D printed personalised medicine in a hospital setting used this method. Led by a collaborative team at University College London, UK, the Universidade de Santiago de Compostela, Spain, and start-up FABRX, chewable isoleucine tablets with personalised dose were compared to standard treatment in paediatric patients with Maple Syrup Urine Disease at the Hospital Clinico Universitario de Santiago de Compostela, Spain. The study proved successful with improved patient adherence rates, and as a consequence, improved bioavailability. This exciting study propelled the field forward, allowing FABRX to build the first desk-top pharmaceutical 3D printer for personalised medicine and small batch manufacture, the M3DIMAKER.

In addition to extrusion-based techniques, researchers are tackling novel formulations to make vat photopolymerisation techniques active players in the race to personalise medicine with 3D printing.
There are many different vat photopolymerisation techniques, defined by their light source. All are based on a bath, or vat, of photosensitive resin containing the drug of choice, that uses photons to polymerise the resin together into the desired 3D model. Stereolithography (SLA) and Direct Light Processing (DLIP) are some favourable techniques in pharmaceutical 3D printing R&D. They use a laser and projector for light sources respectively, directed into the vat of resin as a layer, building the polymerised resin layer by layer. Volumetric 3D printing, a more recently developed 3D printing technique, uses multiple light beams to create a 3D model as a whole object, printing in seconds. In fact, a research group led by start-up company FABRX printed multiple paracetamol-containing tablets in seconds using a lab-based prototype printer. FABRX has also developed a mini vat photopolymerisation 3D printer, using the light from a smartphone to manufacture warfarin printlets, opening up the possibility of printing medicines at home in the future.
Vat photopolymerisation techniques offer high resolution prints and are often used for microneedle mould manufacture for medical devices. It is also used in orthopaedics and dentistry. For pharmaceutics, vat photopolymerisation is useful for drugs with poor thermal stability as printing is carried out at room temperature. Unfortunately, there are only a few non-toxic excipients available that can be used for pharmaceutical 3D printing, limiting use to early research and development in the pharmaceutical field. The resins that are biocompatible tend to have less favourable mechanical properties for pharmaceutically useful disintegration times. More research is needed to fully exploit this exciting manufacturing technique.
Pharmaceutical 3D printing can be scaled up for the mass manufacture of novel dosage forms with advanced release properties. Two companies are making strides in this area, Triastek in China with their Melt Extrusion Deposition technology and Aprecia in the US with their binder jetting, ZipDose, technology.
Binder jetting is based on a powder bed system, with a roller to move fresh powder mix over the printing area while a binder liquid is deposited where the 3D model is being built. Gradually, the model is built from layers of powder glued together, and the final tablets are sieved out of the powder mass. This type of 3D printing is commercially used for the production of fast dissolving oral dispersible tablets with high drug loading. It is also useful for drugs with poor thermal stability as heat is not used. The downside of binder jetting is the some-what wasteful nature of the powder bed and difficulty maintaining cleanliness as a result. Pharmaceutical company Aprecia have managed to overcome this hurdle with an in-built powder recycling system, allowing binder jetting to become the first pharmaceutical 3D printing technique with a regulatory approved medication on the market. Aprecia gained FDA approval in 2015 for their epilepsy medication Spritam (levetiracetam), quoting the high drug loading ability and fast oral dispersion of tablets as benefits from using this manufacturing technique. Their ZipDose technology is now being expanded for more drugs and treatment pathways.
On the other side of the world, Triastek are exploring Melt Extrusion Deposition for mass production, a scaled-up version of the direct powder extrusion technique mentioned earlier. Triastek recently gained FDA approval for their first mass manufactured formulation for rheumatoid arthritis, listing advanced drug delivery control as the main benefits versus other medication available.
THE FIELD AT LARGE
The pharmaceutical 3D printing field is now growing exponentially, with more and more stakeholders getting involved. As mentioned previously, US Company Aprecia is leading the way for mass manufactured, fast dissolving 3D printed formulations. Triastek in China is making bounds with mass manufactured 3D printed formulations with advanced drug delivery control. UK Company FABRX is pushing the personalised medicine and small batch manufacture front. This is alongside hospitals from around the world setting up clinical studies to make this a reality. An example is Gustave Roussy, the leading hospital for cancer research in Europe, who is setting up clinical studies involving hundreds of people to capture data showing the benefits of 3D printed personalised medicine. Big pharmaceutical companies are also embracing the opportunities that this new technology could offer. More and more companies are entering the race to develop 3D printable formulations for personalised medicine and to implement 3D printing in their clinical trial workflows, for cheaper and more efficient batch manufacture.
CLOSING REMARKS
The field now needs new clinical researchers and pharmaceutical industry groups to get involved to help push the advances further, allowing healthcare to fully take advantage of this game-changing technology. By getting involved in clinical studies for more treatment pathways, patients can feel the benefits sooner rather than later.
References
1. WHO (2007). “Promoting Safety of Medicines for Children” http://apps.who.int/iris/bitstream/handle/10665/43697/9789241563437_eng.pdf;jsessionid=B50E7C5F8BDD38080F620D8F8FA25333?sequence=1 accessed 31st October 2022
2. Nunn T (2011). “Age-appropriate formulations - paediatric needs. EMA Workshop on Paediatric Formulations. https://www.ema.europa.eu/en/documents/presentation/presentation-age-appropriate-formulations-paediatric-needs_en.pdf accessed 31st October 2022
3.Zajicek A et al (2013). “A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms” AAPS J. 2013 Oct; 15(4): 1072–1081. doi: 10.1208/s12248-013-9511-5
4. Watanabe et al (2018). “Cost of Prescription Drug-Related Morbidity and Mortality” Ann Pharmacother. 2018 Sep;52(9):829-837. doi: 10.1177/1060028018765159.
5. Watson et al (2021). “Pharmaceutical Compounding: a History, Regulatory Overview, and Systematic Review of Compounding Errors” J Med Toxicol. 2021 Apr; 17(2): 197–217. doi: 10.1007/s13181-020-00814-3
6. Goyanes A, Madla CM, Umerji A, Duran-Pineiro G, Giraldez-Montero JM, Lamas-Diaz MJ, Gonzalez-Barcia M, Taherali F, Sanchez-Pintos P, Couce ML, Gaisford S, Basit AW (2019), Int. J. Pharm (567), 118497; DOI: 10.1016/j.ijpharm.2019.118497
7. Rodríguez-Pombo L, Xu X, Seijo-Rabina A, Ong J.J, Alvarez-Lorenzo C, Rial C, Nieto D, Gaisford S, Basit A.W, Goyanes Á (2022), Additive Manufacturing (52), 102673. DOI:10.1016/j.addma.2022.102673
8. Xu X, Seijo-Rabina A, Awad A, Carlos Rial, Gaisford S, Basit A.W, Goyanes Á (2021), International Journal of Pharmaceutics (609), 121199; DOI:10.1016/j.ijpharm.2021.121199
9. Aprecia PR Newswire (2016), Blue Ash. “FIRST FDA-APPROVED MEDICINE MANUFACTURED USING 3D PRINTING TECHNOLOGY NOW AVAILABLE.”. Accessed January 9, 2023. https://www.multivu.com/players/English/7764551-aprecia-pharmaceuticals-spritam/.
10. Everett, H. (2021, February 10). Triastek receives FDA ind clearance for 3D printed drug to treat rheumatoid arthritis. TRIASTEK RECEIVES FDA IND CLEARANCE FOR 3D PRINTED DRUG TO TREAT RHEUMATOID ARTHRITIS. Retrieved January 10, 2023, from https://3dprintingindustry.com/news/triastek-receives-fda-ind-clearance-for-3d-printed-drug-to-treat-rheumatoid-arthritis-184159/
11. FABRX. (2021). Fabrx and Gustave Roussy enter into an agreement to develop a novel, personalised, multi-drug dosage form for the treatment of patients with early-stage breast cancer. FABRX. Retrieved January 10, 2023, from https://www.fabrx.co.uk/2021/06/25/fabrx-and-gustave-roussy-enter-into-an-agreement-to-develop-a-novel-personalised-multi-drug-dosage-form-for-the-treatment-of-patients-with-early-stage-breast-cancer/