
The pharmaceutical industry is expected to be worth $1.5 trillion by 2023, and the field of pharmacovigilance plays a crucial role in ensuring the safety of drugs. Artificial Intelligence (AI) and Machine Learning (ML) models are being applied to improve the pharmacovigilance process, including case intake using Optical Character Recognition (OCR) and Natural Language Processing (NLP), natural language generation (NLG) for narrative writing, robotic process automation (RPA) for dynamic case workflow, AI-based signal detection, and AI-based adverse event prediction. These advancements have the potential to increase efficiency, accuracy, and consistency in pharmacovigilance, as well as reduce costs and delivery timelines for pharmaceutical organizations.
With rapid digitalization and increased research in the sector, the pharmaceutical industry has experienced a remarkable development in the last three years, opening doors for innovative routes of therapy for humankind. The pharmaceutical industry is expected to be worth $1.5 trillion by 2023, according toestimates.
According to a research published in the Clinical Pharmacology and Therapeutics Journal, AI/ML approaches are being used in multiple areas to support drug development and regulatory submissions as well as facilitating in review and research.
Pharmacovigilance is an essential component of the drug development cycle that ensures pharmaceutical products meet the required safety profile before they are approved for market. It is vital to do post-marketing safety and efficacy studies for most products.
With organizations seeking to optimize their spend through the entire drug life cycle, experts are looking at the use of AI/ML to make the Pharmacovigilance process more effective and efficient.
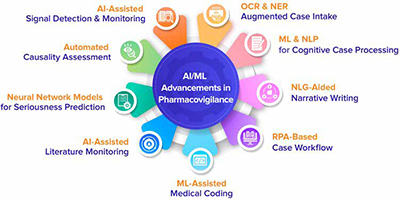
The following are the nine areas in which AI/ML technologies have been utilized to improve the entire pharmacovigilance process:
In pharmacovigilance, case intake refers to the process of collecting and recording information about adverse events associated with the use of a medication or medical device. Case intake is one of the most common applications of AI.
OCR & NER has the potential to extract data quickly and efficiently with the expanding volume of information and multiple data sources like Medical Literature, Social Media, Electronic Health Records, Regulatory Reporting, News Websites and Papers, Mobile Applications, etc. containing primarily unstructured data including free text narratives and scanned PDFs, avoiding the massive amount of time required for human data entry.
OCR is a technology to recognize and extract text from images and scanned documents, such as PDFs or scanned reports. This can be particularly useful in pharmacovigilance, as many adverse event reports are submitted in paper format, and OCR can be used to digitize these reports and make the information they contain accessible to automated systems.
In addition to OCR, NER (Named Entity Recognition) is another technique used in natural language processing that can be applied to case intake to automatically identify and classify relevant entities, such as the name of the drug, the symptoms experienced by the patient, and the outcome of the event. This can help to streamline the case intake process and make it more efficient.
There are various NER techniques and models that can be used for this purpose, such as rule-based systems, statistical models, and neural network-based models.
When using OCR for case intake, the first step is to convert the scanned document or image into a digital format that the OCR software can process. Once the image is in a digital format, OCR software can analyse it and recognize the text it contains. The software will then output the recognized text in a machine-readable format, such as a plain text file or a structured data format like XML or JSON.
The next step is to use NER to extract the named entities such as drug name, symptoms and patient details, etc. from the recognized text. This information can then be stored in a structured format for further analysis and reporting.
Machine learning based cognitive case processing is a method of automating the analysis and triage of adverse event reports in pharmacovigilance using ML algorithms. It involves the use of ML techniques to analyse large amounts of text data, such as patient narratives or adverse event reports, to extract relevant information and classify the reports into different categories.
In cognitive case processing, an ML model is trained on a large dataset of labelled adverse event reports. The model learns to identify patterns and relationships in the data that correspond to different categories of reports, such as serious adverse events or non-serious events.
Once the model is trained, it can be applied to new adverse event reports to automatically classify them into the appropriate categories. This can help to streamline the case processing process and make it more efficient, as well as increasing the consistency and accuracy of the classification.
Additionally, this approach can be enhanced with the use of natural language processing (NLP) techniques to extract entities such as drug names, symptoms and patient details from unstructured narratives which can further improve the accuracy and speed of case processing.
Narrative writing plays a key role in pharmacovigilance by providing a detailed account of an adverse event, enabling better understanding and communication of the event, and helping to identify patterns or trends that may indicate a safety issue.
According to an ICSR research article in their publication, Perspectives in Clinical Research, resourcing, consistency, timeliness with high-quality and potentially various data sources utilised as input, and considerable variation in the templates are all challenges with narrative writing. Furthermore, in situations with several follow-ups, there is a high possibility that the story might become fragmented and confusing.
Using Natural Language Generation (NLG) to automate narrative writing increases the quality and consistency of the narratives while decreasing the time required to construct and complete the narrative. The model can extract all necessary information quickly and effectively, produce the narrative, and place it in the required format or template.
Built-in audit trails and version control can ensure that each version of the narrative is logged and saved for easy access and comparison.
Robotic Process Automation (RPA) is one of the emerging forms of business process automation technology based on the notion of software bots or artificial intelligence.
According to one study published in the Applied Clinical Informatics Journal of the American Medical Informatics Association (AMIA), workflow automation, which entails finding sequences of processes that may be optimised by leveraging technology and modern computing, provides potential to solve quality, safety, and efficiency concerns.
Automation is something pharmaceutical companies are eager to implement to save costs and delivery times. Robotic processes that perform repetitive operations can greatly minimise the need for manual effort by enhancing productivity, compliance, and overall quality while also immediately boosting efficiency.
For case workflows in pharmacovigilance, bringing in rule-based automation, robotic process automation (RPA) or automation based on regular expressions could help improve compliance significantly.
Medical coding is the classification of a number of similar verbatim phrases using a certified medical lexicon that is either given by the client or granted permission by the relevant licencing authorities, in order to provide a statistically quantifiable count of all related terms.
The process of matching a reported term to a dictionary entry can be aided by machine learning. The verbatim words that have a precise match in the dictionary will be automatically coded as the programme runs validation during this process.
ML can reduce the risk of coding errors, as it is not subject to the same types of mistakes that humans can make when coding manually. By automating the coding process, it is possible to reduce the effort and cost associated with manual coding.
Pharmacovigilance professionals seek for indications of drug safety risks in research findings, case studies, and other publications. This process typically takes a long time and is prone to human error. AI-enabled solutions could be used to automate the process by ingesting data from an increasing number of scientific sources and automatically flagging possible drug safety risks.
Literature monitoring in pharmacovigilance could use Artificial Intelligence (AI) and Natural Language Processing (NLP) techniques to automate the process of monitoring scientific literature for new information related to the safety and efficacy of drugs and medical devices.
The process typically involves the use of AI algorithms to search and extract relevant information from scientific journals, conference proceedings, and other sources. This can include identifying new studies related to a specific drug or medical device, tracking changes in the safety profile of a drug over time, and identifying emerging safety concerns.
One of the main benefits of AI-assisted literature monitoring is the ability to quickly and efficiently process large amounts of information from a wide range of sources. This can help to identify potential safety issues early on and can be used to inform the development of new safety measures or to make regulatory decisions about a drug or device.
Additionally, AI-assisted literature monitoring can be used to prioritize articles for review by human experts, focusing on the most relevant studies and publications for a given topic, which can save time and resources for the pharmacovigilance teams.
The seriousness of adverse events is a critical component in determining reporting timelines, and it is usually handled manually by pharmacovigilance professionals. Because of the huge growth in the amount of safety reports, it has become necessary for pharma companies to utilize AI/ML enabled scalable solutions that also fulfil reporting timeline requirements.
A neural network approach can provide a precise and scalable solution for potentially enhancing the assessment of the seriousness of adverse events in spontaneous, solicited, and medical literature reports, according to the findings of a research study that was published in Drug Safety, the official journal of the International Society of Pharmacovigilance (ISoP).
Neural networks can be used to predict the seriousness of AEs reported in pharmacovigilance. These models can take a variety of inputs, such as the patient's demographic information and the details of the AE and use that information to make a prediction about the seriousness of the event.
There are different types of neural networks that can be used for this task, such as feedforward neural networks and recurrent neural networks. In some cases, a combination of both can be used to improve the performance of the model. These neural networks can be trained on large amounts of historical data on AEs to learn the patterns that are associated with serious events.
Once trained, the model can then be used to predict the seriousness of new AE reports as they come in. This can help to quickly identify and prioritize serious events, which can be important for ensuring the safe and effective use of medications.

In pharmacovigilance, causality assessment is the process of determining whether an adverse event is causally related to the use of a specific drug or medical device. Machine Learning (ML) models can be used to automate this process, making it more efficient and accurate.
The WHO UMC causality assessment and the Naranjo causality assessment are two widely accepted and utilised methods for assessing causality worldwide.
Some other specific ML models that can be used for causality assessment in pharmacovigilance include:
The models should be trained with a high-quality, representative, and well-annotated data, and should be validated with independent datasets to ensure the performance and generalization.
The increasing complexity of data reporting and regulators' expectations of being more proactive in detecting adverse events is transforming how signals are gathered and managed.
Proactive signal detection approaches are considered as a part of Good Pharmacovigilance Practices (GVP). More significantly, by including signal detection into their PV monitoring, pharma organizations lower their risks and raise the likelihood of positive outcomes - even when adverse events are identified.
There are several ways that AI and ML can be used to improve signal detection in pharmacovigilance:
Challenges with AI/ML in Pharmacovigilance
There are some challenges with using AI/ML in pharmacovigilance which include:
Artificial Intelligence and Machine Learning have a lot of potential for safety and pharmacovigilance. These technologies have the ability to change the focus of the pharmacovigilance function from data collecting and reporting to assisting in improving product quality, streamlining treatment regimens, reducing costs, and enhancing patient safety.
Agile pharmaceutical organisations may be able to provide enticing alternatives to current procedures and workflows as a result of the shift to AI/ML-based pharmacovigilance solutions. The future of pharmacovigilance is in digitalization, AI analytics, and patient-centred data collection, all of which are expected to increase overall medication safety. To ensure performance and generalisation, the AI/ML models should be trained on high-quality, representative, and well-annotated data and evaluated on separate datasets.
The advantages of implementing AI/ML are visible in the long term. It's time for the pharmaceutical business to advance by fast adapting, creating AI/ML use cases, and implementing them at scale.
References:
1. https://www.worldpharmatoday.com/news/trends-and-estimates-for-the-pharmaceutical-industry-in-2023/
2. Liu Q, Zhu H, Liu C, Jean D, Huang SM, El Zarrad MK, Blumenthal G, Wang Y. Application of Machine Learning in Drug Development and Regulation: Current Status and Future Potential. Clin Pharmacol Ther. 2020 Apr;107(4):726-729. doi: 10.1002/cpt.1771. Epub 2020 Feb 8. PMID: 31925955.
3. Routray R, Tetarenko N, Abu-Assal C, Mockute R, Assuncao B, Chen H, Bao S, Danysz K, Desai S, Cicirello S, Willis V, Alford SH, Krishnamurthy V, Mingle E. Application of Augmented Intelligence for Pharmacovigilance Case Seriousness Determination. Drug Saf. 2020 Jan;43(1):57-66. doi: 10.1007/s40264-019-00869-4. PMID: 31605285; PMCID: PMC6965337.
4. Ledade SD, Jain SN, Darji AA, Gupta VH. Narrative writing: Effective ways and best practices. Perspect Clin Res. 2017 Apr-Jun;8(2):58-62. doi: 10.4103/2229-3485.203044. PMID: 28447014; PMCID: PMC5384400.
5. Jorge Ribeiro, Rui Lima, Tiago Eckhardt, Sara Paiva,Robotic Process Automation and Artificial Intelligence in Industry 4.0 – A Literature review, Procedia Computer Science, Volume 181, 2021, Pages 51-58, ISSN 1877-0509, https://doi.org/10.1016/j.procs.2021.01.104.
6. Zayas-Cabán T, Haque SN, Kemper N. Identifying Opportunities for Workflow Automation in Health Care: Lessons Learned from Other Industries. Appl Clin Inform. 2021 May;12(3):686-697. doi: 10.1055/s-0041-1731744. Epub 2021 Jul 28. PMID: 34320683; PMCID: PMC8318703.
7. https://www.grandviewresearch.com/industry-analysis/artificial-intelligence-ai-healthcare-market
8. Routray R, Tetarenko N, Abu-Assal C, Mockute R, Assuncao B, Chen H, Bao S, Danysz K, Desai S, Cicirello S, Willis V, Alford SH, Krishnamurthy V, Mingle E. Application of Augmented Intelligence for Pharmacovigilance Case Seriousness Determination. Drug Saf. 2020 Jan;43(1):57-66. doi: 10.1007/s40264-019-00869-4. PMID: 31605285; PMCID: PMC6965337.
9. https://www.who.int/publications/m/item/WHO-causality-assessment
10. https://en.wikipedia.org/wiki/Naranjo_algorithm
11. Ball R, Dal Pan G. "Artificial Intelligence" for Pharmacovigilance: Ready for Prime Time? Drug Saf. 2022 May;45(5):429-438. doi: 10.1007/s40264-022-01157-4. Epub 2022 May 17. PMID: 35579808; PMCID: PMC9112277.
12. Islam MS, Hasan MM, Wang X, Germack HD, Noor-E-Alam M. A Systematic Review on Healthcare Analytics: Application and Theoretical Perspective of Data Mining. Healthcare (Basel). 2018 May 23;6(2):54. doi: 10.3390/healthcare6020054. PMID: 29882866; PMCID: PMC6023432.
13. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/good-pharmacovigilance-practices-and-pharmacoepidemiologic-assessment
14. https://www.fda.gov/news-events/press-announcements/fda-releases-artificial-intelligencemachine-learning-action-plan