Advanced Model Predictive Control System of Continuous Biopharmaceutical Manufacturing Process
Ravendra Singh, C-SOPS, Department of Chemical and Biochemical Engineering, Rutgers, The State University of New Jersey
The progressive acceleration of continuous bioprocessing method has become the focal point of advanced research and study for the biotech companies in recent years. Implementation of this method is a tedious and lengthy process but has been proven beneficial down the line. Advancement of this methodology is initiated by the U. S. Food and Drug Administration (FDA) through introduction of Quality by Design (QbD) approach. In parallel, sophisticated bioreactors are now available in the market with various applications to support the continuous upstream process. In motion, monoclonal antibodies (mAb’s) are produced by studying the behavior of CHO cell culture, affected by different media and controlled variables. However, the control of continuous biomanufacturing process is still a challenging task because of different level of complexities. In this work, an advanced model predictive control (MPC) system has been developed for continuous biomanufacturing process. The control relevant process models have been developed using experimental data. A PID based feedback control system has been also developed. The performance of MPC based control system has been compared with PID based control system.
Introduction
Currently, the bio pharmaceutical industry is going under a paradigm shift from batch to continuous manufacturing (CM). One of the main advantages of CM is that it provides a suitable manufacturing platform to enable real time monitoring and control of critical process parameters (CPP’s) and critical quality attributes (CQA’s). In the last decade, the PAT tools are studied in detail for pharmaceutical manufacturing. Different types of chromatography and spectroscopy technologies are used to monitor the CQA’s of biopharmaceuticals. However, much less attention has been paid to the real time control of CPP’s and CQA’s using the feedback model predictive control mechanism.
The objective of this work is to demonstrate the design and development of a model predictive control (MPC) strategy for continuous biomanufacturing processes.
Design and evaluation of the control system
This study is focused on design and evaluation of advanced control system for upstream biomanufacturing process. The manufacturing process has been previously described. For control of pH, dissolved oxygen (DO) and temperature, the bioreactor uses inbuilt Proportional-Integral (PI) controller. In addition to that, a supervisory control system has been developed for control of any additional parameters required for the process. The supervisory control system provides the setpoint of the local level control system and thereby linking the CQA’s with operating parameters.
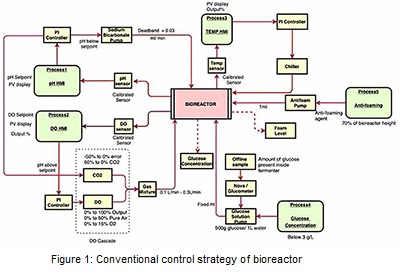
The control architecture of the bioreactor is shown in Figure 1. In the first layer of the control system, the pH is monitored using an inbuilt electrode sensor and controlled utilizing a PI controller. The actuator, in this case, is the speed of the pump that feeds base materials. For this loop, the controller acts according to two different conditions i.e., whether the pH is below the setpoint or pH above the setpoint. When the measured pH is below the setpoint, the base (e.g., sodium bicarbonate) is added into the system via inbuilt pump. On the other end, when the measured process pH value is above the setpoint, CO2 gas is added to the system via gas chamber. The next control variable dissolved oxygen (DO) is monitored using an inbuilt ‘polarographic sensor’ and is controlled using the PI-based controller by manipulating the gas flow rate. In DO cascade, air and O2 gas are added into the system via the gas chamber. The amount of these two gases added into the system depend on the DO controller output percentage (%). The total gas mixture of all the 3 gases (air, O2, CO2) combined should be 100%. Once the gas mixture is occupied in the gas chamber, gases are then entered into the system via an inbuilt multi flowmeter. DO and pH control loops are highly interactive. The temperature is monitored using the inbuilt platinum resistance temperature detector (RTD) sensor and controlled through a PI controller by manipulating the temperature of fluid flowing through the jacket of the bioreactor. The closed-loop model has been developed and apply to evaluate the performance of these control loops. The advanced model predictive control (MPC) system has been developed and implemented into the model for performance evaluation.

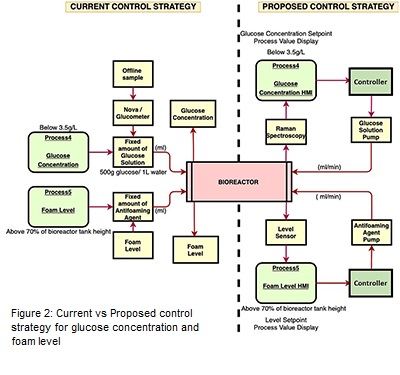
Glucose concentration and foam level are conventionally controlled manually. Offline sample from the bioreactor was analyzed using nova analyzer/ glucometer (glucose oxidase method) to find out the concentration of glucose. If the glucose concentration was less than the desired setpoint, mass balance was carried out to find out the amount of glucose solution needed for the total volume at that time. Simultaneously, fixed volume of glucose solution would be added to the system via inbuilt pump at a fix flowrate. For foam level control, in person observation is currently needed to constantly keep a track on the level which is resource intensive. Whenever the foam level increases above 70% of the bioreactor’s height, immediately 1ml of antifoaming agent is added to the system via inbuilt pump. Pictorial representation of this strategy is reflected in Figure 1. A novel control strategy has been developed and evaluated for controlling of glucose concentration and foal level as discussed in next section.
Proposed strategy for Glucose Concentration and Foam Level control
Control strategy for glucose concentration and foam level has been proposed in this work to convert them into feedback control loops for the aim of achieving the complete automated upstream bioprocess. For glucose, on-line monitoring of glucose concentration can be achieved using Raman spectroscopy. Once the process value of glucose concentration is generated from Raman spectroscopy, the data can be transferred to the HMI where the error would be calculated in reference to the desired setpoint. If the glucose concentration is less than the given setpoint, controller will automatically turn ON the pump to release the required amount of glucose solution into the system by manipulating the pump flowrate. The actuator in this case is the flowrate of the pump. The foam level control will follow the same steps. The only difference here would be that the level indicator probe will be inserted into the bioreactor via head plate port for in-line monitoring of the level. In Figure 2, the pictorial representation of the current versus proposed control strategy of glucose concentration and foam level is displayed.
Integrated closed loop flowsheet model of bioreactor
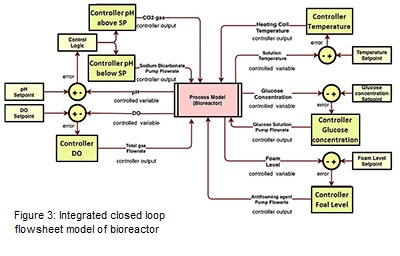
The integrated closed loop flowsheet model of bioreactor has been developed as shown in Figure 3. The pH feedback control loop has been developed using two process models. One process model has been used when the CO2 is the input (pH is above the setpoint) and other process model has been used when the sodium bicarbonate is the input (pH is below the setpoint). For each of these models, one controller has been added. A control logic has been developed using math function in Simulink (Mathworks) which states that when the error is positive, the sodium bicarbonate pump will be active for manipulating the flowrate. On the other hand, when the error is negative, the flow rate of CO2 gas flowing through the gas chamber will be manipulated. Both these models have been added using an addition block and further divided by two to get the actual value of the controlled pH which is further compared by the setpoint value to generate the error. The DO feedback control loop is comparatively more complicated than the pH loop. Currently the closed loop has been designed in a way where the input to the process model is the total gas flowrate which include O2 and air gas and the output is the controlled DO values. The temperature feedback control loop follows the same design as shown in figure 3. The feedback control loop design for glucose concentration and foam level has been also shown in Figure 3. Both, PID and MPC controller has been implemented in the model to evaluate its performance.
Implementation of the control system via distributed control system (DCS)
For implementation of the control system, the pilot-plant need to be integrated with the distributed control system. The integration strategy of the bioreactor with distributed control system (DCS) is shown in Figure 4. For this integration, there is a need for multi-layer communication between different software and hardware. In Figure 4, the integration of DeltaV (Emerson), a DCS platform with the bioprocess is shown. The first step is the communication of DeltaV PC with an OLE process control (OPC) Kepware server 1 via OPC data access (DA) connection. Kepware OPC is a connectivity platform enabling communication between two systems. DA connection provides data from different data sources to the server client. Kepware server 1 is connected with another Kepware server 2 via OPC Unified Architecture (UA) connection. OPC UA is a standard that ensures open connectivity, security, and reliability of automated systems. OPC server 2 essentially communicates with bioreactor (Eppendorf) command PC which is a communication platform to operate the bioreactor. Eppendorf itself communicates with the Nova BioFlex2 Embedded PC via DA/ Extensible Markup Language (XML) connection. The focus of developing this integration strategy is to implement the control strategy of the bioreactor in DCS to control the CPP’s and CQA’s via this multilayer communication protocol

Results and Discussions
The performance of the control system has been evaluated for set point tracking and disturbance rejection. The pH control loop has been considered here for demonstration purposes. In the Figure 5, the performance of the PID and MPC controller is compared where in one case (a), the pH is changed from 7.10 to 7.15 pH. In this case, the PID controller takes approximately 35 minutes to reach to the new setpoint whereas the MPC controller takes 15 minutes to reach to the new assigned setpoint. The flowrate of the pump for both controllers remains steady at 0.235 ml/min after reaching the setpoint. The value of flowrate can vary depending on the step change provided to the system. In the other case (b), the pH is changed from 7.10 to 7.07. In this case, the CO2 gas needs to be added to the system to decrease the value of pH. The Figure 4 shows that the MPC completes the step change action in 5.5 times lesser time in compared to PID controller. As expected, the sodium bicarbonate pump remains off throughout the operation. The response of CO2 gas (actuator) for both controllers is also shown in the figure. Similarly, the performance of other control loops has been evaluated.

Conclusions
An advanced model predictive control (MPC) system has been developed for bioreactor used in continuous manufacturing. A validated integrated closed-loop flowsheet model has been developed as well. The performance of the PID and MPC for setpoint tracking and disturbance rejection has been compared and it has been observed that the MPC perform better. A systematic framework to implement the control system in the biomanufacturing process has been proposed.
Acknowledgements
This work is supported by the U. S. Food and Drug Administration (FDA) through FDA-CBER Award Number1R01FD006588.
References
1. Yu, L. (2016). Continuous Manufacturing Has a Strong Impact on Drug Quality. U.S. Food and Drug Administration (FDA). https://blogs.fda.gov/fdavoice/index.php/2016/04/continuous-manufacturing-has-a-strong-impact-on-drug-quality/
2. Chopda, V., Gyorgypal, A, Yang O., Singh, R., Ramachandran, R., Zhang, H., Tsilomelekis, G., Chundawat, S., Ierapetritou, M. (2021). Recent Advances in Integrated Process Analytical Techniques, Modeling, and Control Strategies to Enable Continuous Biomanufacturing of Monoclonal Antibodies. Journal of Chemical Technology and Biotechnology. https://doi.org/10.1002/jctb.6765.
3. Gyorgypal, A.; Chundawat, S. P. S (2022). Integrated Process Analytical Platform for Automated Monitoring of Monoclonal Antibody N-Linked Glycosylation. Anal. Chem., 94 (19), 6986–6995. https://doi.org/10.1021/acs.analchem.1c05396.
4. Tiwold, E. K.; Gyorgypal, A.; Chundawat, S. P. (2022). Recent Advances in Biologic Therapeutic N-Glycan Preparation Techniques and Analytical Methods for Facilitating Biomanufacturing Automation. ChemRxiv (Cambridge Cambridge Open Engag. 2022. https://doi.org/10.26434/chemrxiv-2022-sdq2g.
5. Singh, R., Sahay, A., Karry, K. M., Muzzio, F., Ierapetritou, M., Ramachandran, R. Implementation of a hybrid MPC-PID control strategy using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot-plant. International Journal of Pharmaceutics. 2014b; 473, 38–54.
6. Bhaskar, A., Barros, F. N., Singh, R. (2017). Development and implementation of an advanced model predictive control system into continuous pharmaceutical tablet compaction process. International Journal of Pharmaceutics, 534 (1-2), 159-178. https://doi.org/10.1016/j.ijpharm.2017.10.003.











