
The pharmaceutical manufacturing industry is different from other industries in that it is concerned with human health and follows a highly sensitive and critical operating scenario, aiming to produce pharmaceutical drugs for public treatment. The pharmaceutical manufacturing industry has until now, followed a batch processing scenario to ensure that each batch was produced efficiently and effectively, conforming to its pre-planned specifications and in compliance with cGMP and regulatory requirements. A particular quantity of a medication or other substance that is produced in accordance with a single production order and is produced during the same manufacturing cycle is referred to as "batch processing" in regulations like the FDA's Code of Federal Regulations (21 CFR) 210.31. To put it another way, it entails the step-by-step assembling of various product components to produce the finished manufactured product.
In batch processing, a subsequent batch can be processed only after the current batch has been completed. Continuous pharmaceutical manufacturing [CM] has become increasingly popular in recent years, but the transition from batch to continuous manufacturing is still under evaluation.
Continuous manufacturing [CM] is a production scenario that uses the continuous supply of raw materials directly within the manufacturing process at the same facility without stopping and/or shutting down until completion.
In this article, we shed light on a new recent scenario for producing pharmaceutical products and the one currently in place, discuss the challenges and pros and cons of each, and make comments on them.

Pharma manufacturing industries are complicated and depend on several factors, such as capital expenditures ("CAPEX") that include infrastructure, supplied resources, buildings, equipment, machines, etc., in addition to giving a great deal of attention to operation expenditures ("OPEX") to assure the effect of planning, doing the proper feasibility studies, and analyzing the relevant market trends, including competent personnel contributions as decision makers to ensure successful production operations.
Pharma manufacturing needs to include consumer demands, industry competitions, manufacturing legal requirements, and the correct implementation of a quality management system (QMS) to ensure the smooth running of manufacturing processes and relevant integrated processes, aiming to get the product as per the approved specifications, fit for its intended use, and attaining the best possible return on investment (ROI) for the sake of the stakeholders.
As per recent market trends, the pharmaceutical industry loses about $50 billion due to the shortcomings of batch processing as a result of time constraints, delivery issues, damage, or the cost of a review.
Senior management should pay close attention to the analysis of the factors and attributes controlling the manufacturing processes before deciding which manufacturing method is reliable and appropriate for their manufacturing methodology, taking into account applying and adhering to all relevant pharma legislation and regulation and coming in at the bottom of the list.
Continuous manufacturing and batch processing are two sides of the same coin. Both are used in different manufacturing industries, "especially pharmaceutical manufacturing," provided that they consider the relevant regulations and requirements aiming to produce pharmaceutical drugs as per the approved specifications, in compliance with cGMP regulations, and fit for their intended use.
Manufacturers have been producing pharmaceutical products using batch processing for many years. A batch is defined in the FDA’s Code of Federal Regulations (21 CFR) 210.3 as a certain amount of a drug or other material that is intended to have a uniform character and quality within defined limits and is manufactured according to a single production order during the same manufacturing cycle.
Multiple components of a drug are mixed in a single step during batch processing. Each stage of the creative process requires a break from batch processing. It takes multiple stages [in terms of time, money, and labour] as the materials go from step to step to process the current batch before the next batch can be processed.
The European Medicines Agency's EMA ICH Q7 defines a batch in batch processing as a homogeneous material that falls within specific parameters. According to EMA ICH Q7, a batch can correspond to a specific percentage of the production in the event of continuous manufacturing. The batch size can be defined both as a fixed quantity and as a fixed time interval.
In continuous manufacturing, where a batch can be based on a defined amount of product or raw material, a fixed time period, or a timeframe in production, the FDA has declared that the batch and batch definitions from 21 CFR 210.3 apply.
Due to factors like COVID-19 PANDEMIC, which highlighted the vulnerability of global supply chain networks, including those supporting the pharmaceutical industry, to large external shocks, in addition to Brexit and a push for sustainability, some manufacturers have transitioned to continuous manufacturing.
Early in the crisis, labour and shipping problems forced nations to scramble to maintain access to life-saving medications, raising concerns about the pharmaceutical industry's long-term viability as climate change and mounting geopolitical tensions weigh heavily on the world.
The phrases "continuous processing," "continuous manufacturing," and similar expressions are employed. However, they cannot be used interchangeably because they have different meanings. The phrase "continuous production" refers to a production schedule that operates constantly throughout the whole week. A single-unit operation is referred to as "continuous processing" if raw materials are continually loaded, processed, and discharged without pause.
Batch manufacturing (BM), the primary method of production, uses separate unit operations with offline quality testing and storage between each stage. It is characterized by the sequential management of the arrangement of tanks for each process. It is used in the pharmaceutical and fine chemical industries.
While manufacturers are willing to maximize profits, their main reason for using continuous manufacturing is its potential to reduce manufacturing costs, making them more competitive in the market and increasing their ROI.
A comparison of the net present values of investing in CM versus batch manufacturing in a research study funded by the US Food and Drug Administration (FDA) provides the most comprehensive analysis to date on this issue. The analysis demonstrates that the economics of investing in CM versus batch facilities is not only a winning investment strategy but also one that can reduce disruptions in the flow of pharmaceutical products from global risk events.
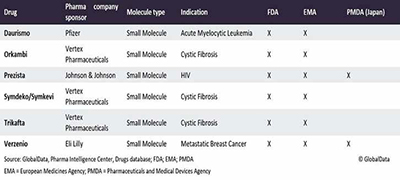
Continuous manufacturing is a recent and growing model used in the pharmaceutical manufacturing industry. It has recently gone from being a trendy idea to a reality. Given that the number of continuous manufacturing facilities under review has expanded in the last five years, see the table below. Continuous manufacturing (CM) is the use of a continuous flow of raw materials via continuous manufacturing processes using a single unit operation to produce huge quantities of products without stopping. It is distinct from batch processing, which employs starting materials in a sequential process with breaks in between batches. CM processes, which are used for facilities producing oral solid dosage (OSD) pharmaceutical products, will replace the current batch processing technology that is more labor-intensive, less efficient, and more likely to result in product defects.
Continuous manufacturing (CM) is a process that combines a number of unit operations together. It is continuously processing raw materials to create the finished product via a series of procedures that maximize drug production to maintain a steady stream of medications.
Within continuous manufacturing [CM], it is important to be able to systematically understand what is happening in your process, find out what is happening at your continuous manufacturing process, and be ready for an explanation at the time of an authority inspection.
To satisfy regulatory bodies concerning continuous manufacturing, especially at an elevated level of traceability, companies need to establish smart factories for continuous manufacturing using AI, industry 4, pharma 4, and machine learning (ML) with data from IIoT sensors, planning to satisfy customer needs for their standard and customized products that enable companies to improve their bottom line by becoming more productive, competitive, and profitable. Continuous manufacturing will play a significant role in the years to come. Despite continuous manufacturing's slow acceptance, the FDA is in support of a wider adoption of these technologies.
Manufacturing processes and process analytical technologies are the two main components of continuous manufacturing that require control (PAT). What aspects of the manufacturing processes are essential, and what particular difficulties arise in the ongoing production of medicinal substances? Important process parameters (CPPs), important material attributes (CMAs), and important quality parameters (CQAs) are monitored and regulated by them. By eliminating work-up unit operations, it streamlines manufacturing processes. It is usually housed in a single building and has smaller hardware.
Small molecules used in pharmaceuticals are subject to continuous manufacturing (CM), which is also applicable to personnel medications. Maintaining quality throughout an operational line in continuous manufacturing enables rapid response to alerts or recommendations. It makes sure that the control, monitoring, and output are all consistently within specs.
For companies to be transition from batch processing to continuous manufacturing, they need to have extensive manufacturing knowledge and equip their facilities with competent production, engineering, maintenance, financial, quality, and safety staff who will play crucial roles in continuous manufacturing operations. They need to keep an eye on the method of linking the used technology, software automation, and smart automated equipment during the implementation duration. They need to focus on the optimization of the production process by saving the required, proper, efficient, and sufficient resources to create, store, track, and check asset data during continuous manufacturing operations. Continuous manufacturing makes effective use of the resources within the manufacturing facility, such as machines, energy consumption, and infrastructures such as utilities (such as HVAC systems and water treatment systems), which are major utilities.
In continuous manufacturing (CM), you need to allocate the proper starting materials all the time to meet the quality or potency requirements, always considering risk management assessment, doing the necessary validation, and controlling product quality attributes throughout all the operation phases. You need to adapt and use Effective, accurate, precise, and efficient planning for an alternate route is needed in case there is equipment downtime and/or failure, which is considered a primary route need during implementation to avoid loss and failure of manufacturing continuous operation and to keep manufactured product specifications within the acceptance criteria.
In continuous manufacturing (CM), employees are less involved in tracking procedures and guaranteeing quality, so you need to have the skilled and qualified personnel required during production operations, which costs more in personnel fees. Use cutting-edge AI-based solutions that enable facilities to use continuous manufacturing while still custom-designing the product.
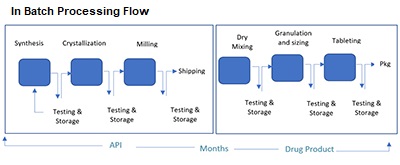
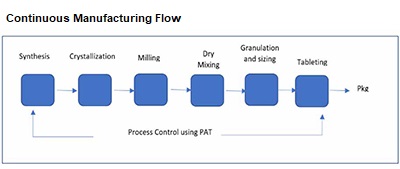
Batch processing, which enables product tracing to the original batch, is considered a valuable tool in product tracing and tracking for investigation and the recall process, if required. In continuous manufacturing, to track and trace items, you need to use batch numbers to track items and, if required, recall them by creating a paper trail for them.
There are many industries that use continuous manufacturing to reduce costs and get more concise finished products, such as automotive, metals and mining, biotechnology, and pharmaceuticals.

In order to enhance product quality, reduce product faults, and ease shortages, FDA officials in the United States have been urging firms to convert from batch production to continuous manufacturing for years. According to the findings of a recent FDA audit, applications relying on continuous manufacturing methods received approval more quickly and generated more income.
The International Council for Harmonization (ICH) has adopted its guidelines on continuous manufacturing (CM), embracing more modern modes of manufacturing. In the last version of guidance, ICH acceded to the industry’s request to clarify the state of control and process dynamics.
ICH announced the final version of the Q13 guidance and releases on November 16, 2022. To transition from batch processing to continuous manufacturing, manufacturers must first understand the differences between the two and the benefits and drawbacks of each. They must also consider the following factors before moving forward with continuous manufacturing:
You need to define the development consideration with a concentration on process description. Control strategy includes state of control, starting materials, equipment, uniform quality and character of product, product collection or rejection, process monitoring and sampling, risk assessment, failure modes, scale-up, and specifications. When the process is used to make clinical, bioequivalence, or registration stability, the terms comparability and bridging are used. Process Validation and Verification and Lifecycle Management Stability considerations include representative stability batching, stability consideration at scale-up, and site changes or technology transfer in addition to considering the location of information in a regulatory submission.
High and thorough coordination is required for quality and cGMP considerations, including batch release, start-up and shutdown procedures, state of control (product collection and in-process sampling), process validation and continuous process validation, materials traceability in continuous train, stability of starting materials and in-process materials, detection and treatment of non-conformity, personnel procedures and training, materials carry-over, diversion and yield calculation, and production floor product monitoring. Starting materials variability, cleaning validation, equipment breakdown, and failure.
ICH Guidelines: International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use On November 16, 2022, the ICH harmonized guideline for continuous manufacturing of drug substances and drug products Q13 final version was adopted, with the goal of
US FDA Recommendations: The FDA Guidance for Industry PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance clearly notes that the adoption of a scientific risk-based approach to process design may result in the introduction of continuous processing as one of the results. Control procedures that incorporate real-time quality evaluation that is at least equivalent to, or better than, laboratory-based testing on collected samples are made possible by process understanding, control strategies, and on-line, in-line, or at-line monitoring of essential quality attributes.
FDA Guidance on Process Validation and Continuous Verification: According to FDA Guidance on Process Validation and Continuous Verification, processes that involve process validation are in line with a product lifecycle paradigm. At every level of the lifespan of the manufacturing process, the advice promotes the application of contemporary pharmaceutical development principles, quality risk management, and quality systems. The lifecycle idea ties together the development of products and processes, the certification of commercial manufacturing processes, and the upkeep of processes in a controlled state during ordinary commercial production. This advice uses strong science to promote process innovation and development, including continuous manufacturing.
ASTM Standard: ASTM E2537 Validation: Continuous Quality Verification (CQV) is an approach to process validation where the performance of a manufacturing process (or that of a supporting utility system) is continuously monitored, assessed, and adjusted, as necessary. This approach is described in the ASTM Standard: ASTM E2537 Validation: Standard Guide for the Application of Continuous Quality Verification to Pharmaceutical and Biopharmaceutical Products. An approach based on science is used to confirm that a process can create products that consistently meet certain critical quality requirements (CQAs). With real-time quality assurance (which CQV will offer), manufacturing is continuously assessed to guarantee the appropriate quality attributes. Data from production batches can support validation with each manufacturing batch by reflecting the overall system design idea and serving to validate the process.
EU Guidelines: In the European Union, the ICH Guidelines previously mentioned are applicable. The Guidelines for Process Validation, which introduce the idea of continuous process verification, the Guidelines on NIR because it is frequently used as a Process Analytical Technology (PAT) tool for process monitoring and/or control, and the Guidelines on Real-Time Release Testing are three EU Guidelines that may be particularly pertinent to continuous manufacturing. Real-time release testing is frequently combined with continuous manufacturing, although it is not necessary (RTRT). A PAT team was also established by the European Medicines Agency in 2003 to support PAT and QbD initiatives inside the EU. The teams serve as a venue for communication between the Good Manufacturing Practice/Good Distribution Practice Inspectors’ Working Group, the Quality Working Party, and the Biologics Working Party.
Conclusion:
In summary, global and regional regulations, guidelines, and standards are supportive of innovative pharmaceutical development and manufacturing approaches and the consideration of continuous manufacturing operations as experience is gained.
References:
1. https://database.ich.org/sites/default/files/ICH_Q13_Step4_Guideline_2022_1116.pdf
2. ICH Quality documents Q3,Q7,Q8 Q8(R2),Q9,Q10,Q11
3. FDA: Code of Federal Regulations, Part 210 — Current Good Manufacturing Practice in Manufacturing, Processing, Packing, Or Holding of Drugs
4. EMA: ICH Topic Q 7: Good Manufacturing Practice for Active Pharmaceutical Ingredients. Note for Guidance on Good Manufacturing Practice for Active Pharmaceutical Ingredients (Cpmp/Ich/4106/00)
5. FDA: Quality Considerations for Continuous Manufacturing Guidance for Industry, Draft Guidance
6. FDA Guidance for Industry PAT A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance
7. EU Guidelines for Process validation
8. ASTM Standards E2537
9. 21CFR references are in the Glossary section.
10. 21CFR210.3 , 21CFR211.118